Case Report Volume: 1 Issue:1
Abstract
Introduction: Epilepsy surgery emerges as a treatment option in cases of refractory epilepsy, the choice of anesthesia technique will depend on the patient's comorbidities, surgical technique, and the use of neuromonitoring and/or electrocorticography (ECoG).
Clinical Case: A 6-year-old female preschooler diagnosed with Tuberous Sclerosis, resulting in refractory epilepsy with severe neurodevelopmental delay, scheduled for left temporal craniotomy with resection of the left temporal tuber with ECoG under total intravenous anesthesia.
Discussion: The choice of drugs for maintaining anesthesia should be considered based on their ability to modify cortical electrical activity, thereby complicating the localization and delineation of the epileptogenic focus and thus impacting the patient's prognosis.
Conclusion: TIVA remains the cornerstone in neurosurgical anesthesia as it does not interfere with intraoperative electrophysiological monitoring. In adults, the anesthetic technique used is the so-called "asleep-awake-asleep"; however, the patient's age and neurodevelopmental delay ruled her out as a candidate for this anesthetic technique. Upon reviewing the literature, we found few reported cases with characteristics similar to our patient.
Keywords: refractory epilepsy, total intravenous anesthesia, neuromonitoring, pediatric anesthesia
Abbreviations
TIVA, Total intravenous anesthesia; EEG, electroencephalogram; CBD, cannabidiol; ECoG, electrocorticography
Introduction
Tuberous sclerosis is a genetic disorder with autosomal dominant inheritance that manifests with tumor-like lesions in the brain, eyes, heart, lungs, liver, and kidneys. This condition is caused by a mutation in the TSC1 gene located on chromosome 9q34 or the TSC2 gene located on chromosome 16p13.1
Vogt's triad, which includes intellectual disability, seizures, and skin lesions, is characteristic of this condition.2 Cortical tubers are benign hamartomatous lesions associated with epilepsy, intellectual disability, behavioral disorders, and neurological deficits. Seizures are the most common symptom and usually begin around the 4th to 5th month of life.1 There is a correlation between the number and volume of cortical tubers, the age of seizure onset, and the degree of intellectual disability: the earlier the cortical tubers appear, within the first months of life, the earlier seizures begin, and consequently, the greater the degree of intellectual disability.2
Most patients will become drug-resistant and candidates for epilepsy surgery. Before surgery, non-invasive studies are used to identify a "pacemaker tuber," and extended lesionectomy is the treatment of choice to control epilepsy and support the maturation of the developing brain.1
Maintaining anesthesia in neurosurgery presents a challenge for the anesthesiologist, who must integrate factors related to the surgical procedure, the use of tools such as intraoperative neuromonitoring, and patient-related factors including comorbidities and clinical symptoms. TIVA emerges as a favorable option due to its advantages over halogenated agents in terms of brain relaxation, electrophysiological monitoring, neuroprotection, anesthetic recovery, and postoperative nausea and vomiting.3
Clinical case
A 6-year-old female preschool patient weighing 22.5 kg and measuring 112 cm in height, firstborn, with no significant perinatal history, began experiencing her condition at 6 months of age with a generalized tonic-clonic motor seizure lasting 30 minutes, followed by a postictal state with no improvement, accompanied by vomiting and urinary incontinence. She developed status epilepticus, which was controlled with intravenous treatment (medications used are unknown). An EEG showed epileptogenic foci, and treatment was initiated with magnesium valproate 400 mg every 12 hours and prednisone 5 mg every 8 hours.
At one year of age, a diagnosis of tuberous sclerosis was confirmed due to the presence of hypopigmented macules, multicystic renal dysplasia, and cortical tubers.4 During follow-up, an increase in seizure frequency was reported, up to 20 episodes per day over 5 years, without response to various treatments including: vigabatrin 125 mg every 12 hours, clobazam 10 mg every 8 hours, levetiracetam 1.5 g every 12 hours, and magnesium valproate 400 mg every 12 hours, with no adequate seizure control achieved. She was eventually treated with CBD oil at a maximum dose of 6 ml every 12 hours and placed on a ketogenic diet, resulting in a reduction of seizures from 20 episodes/day to 7 episodes/day over 2 months.
Due to recurrence, surgical treatment was decided. Previous EEGs identified the “pacemaker tuber,” and a left temporal craniotomy with resection of the left temporal tuber was performed using ECoG under TIVA.
During the preoperative evaluation, the aforementioned history was confirmed. Airway assessment showed Patil-Aldreti Grade I, adequate oral opening, full dentition, no macroglossia, micrognathia, retrognathia, or prominent incisors; the temporomandibular joint was mobile without neck movement limitations. Mallampati and Bellhouse-Dore assessments were not possible. Global developmental delay was observed according to the Denver II Scale, indicating severe delay (developmental level estimated at 6 months). Labs showed normocytic normochromic anemia; blood chemistry, serum electrolytes, and coagulation times were within normal parameters (Table I).
|
HB |
11.8 g/dL |
Glu |
125 mg/dL |
|
Hct |
35.90% |
BUN |
12 mg/dL |
|
WBC |
5.9 K/uL |
Cr |
0.3 mg/dL |
|
PLT |
171 K/uL |
Na |
138.3 mmol/L |
|
PT |
11.4 seg |
K |
3.8 mmol/L |
|
PTT |
30.2 seg |
Cl |
107.6 mmol/L |
|
INR |
1.04 |
Ca |
9.2 mmol/L |
Pre-anesthetic medication was administered intravenously with omeprazole 1 mg/kg (22 mg), ondansetron 0.15 mg/kg (3.5 mg), and dexamethasone 0.15 mg/kg (3.5 mg); benzodiazepines were avoided as they reduce cerebral electrical activity, hindering ECoG readings and the identification of the epileptogenic focus.5 Due to her neurocognitive condition and limited cooperation, sevoflurane at 1 vol% and 4 L of O₂ was used to facilitate placement of basic non-invasive monitoring. Initial vital signs were: blood pressure 120/65 mmHg, heart rate 71 bpm, respiratory rate 22 bpm, and oxygen saturation 98%. A Radical-7 and SedLine monitor were placed on the right frontal region according to the planned surgical approach (Figure 1), showing unconsciousness readings in R1, R2, and L2.
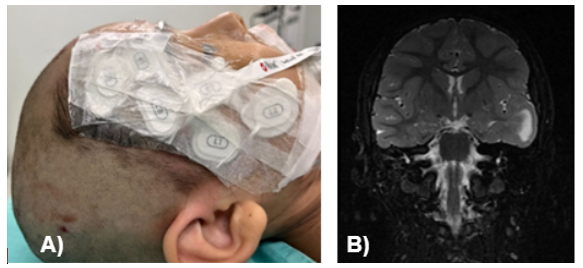
Figure 1 Patient during perioperative period and preoperative MRI.
A, Placement of Sedline sensor; B, Preoperative brain MRI, pacemaker lead highlighted.
Induction was performed using sufentanil 0.5 ng/kg (10 mcg), sevoflurane 2–3 vol%, and rocuronium 0.6 mg/kg (13 mg), achieving SedLine values of 38–40 and a predominance of delta and alpha waves. Orotracheal intubation was successful on the first attempt using a size 4.5 cuffed tube, with proper lung field verification. Three peripheral IV lines, a left radial arterial line, Foley catheter, and a left subclavian central venous catheter guided by ultrasound were placed. The neurophysiologist then installed the neuromonitoring system.
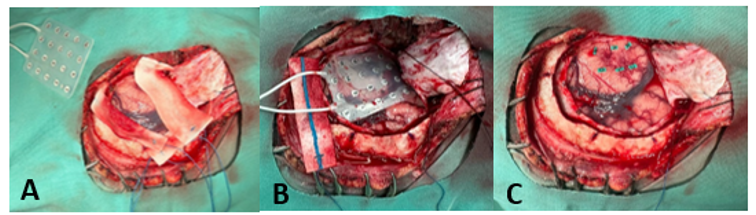
Figure 2 Surgical site. A, Mesh; B, Mesh placed on temporal cortex; C, Marking of areas 12, 7, 8, 14, 9 for tuber resection.
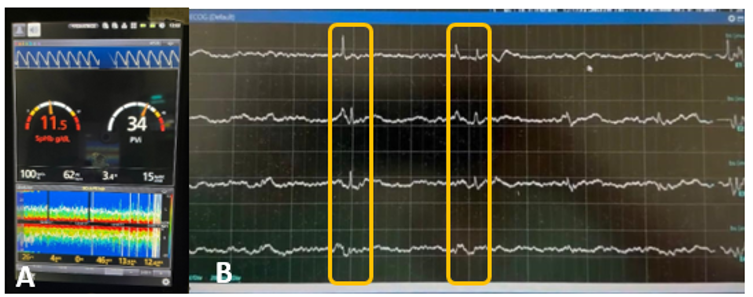
Figure 3 Neuro monitoring. A, Intraoperative Sedline; B, Electrocorticography recording highlighting the epileptic foci.
With both invasive and non-invasive anesthetic monitoring, maintenance anesthesia was achieved using dexmedetomidine at 0.3 mcg/kg/h, with initial target plasma concentrations of propofol at 3 mcg/ml and sufentanil at 0.3 ng/ml.
Intraoperatively, the patient remained hemodynamically and metabolically stable. Anesthesia was maintained with dexmedetomidine (0.3 mcg/kg/h) and titrated concentrations of propofol up to 6.5 mcg/ml and sufentanil up to 0.6–0.7 ng/ml. A left temporal craniotomy was performed, revealing a firm tuber with cortical telangiectasias and volume increase. A mesh (Figure 2) was placed over the temporal cortex, and intraoperative corticography showed spike-and-wave activity in electrodes 12, 7, 8, 14, and 9 (Figure 3). A block resection was performed, and post-resection corticography showed no epileptogenic activity.
At the end of surgery, propofol concentration was 4 mcg/ml, sufentanil 0.4 ng/ml, and dexmedetomidine 0.2 mcg/kg/h. Extubation was carried out without complications. For postoperative analgesia, acetaminophen 15 mg/kg (330 mg) and ketorolac 0.2 mg/kg (12 mg) were administered.
Surgical time was 4 hours 25 minutes, and total anesthesia time was 7 hours 5 minutes, with a urine output of 280 ml (1.8 ml/kg/h) and estimated blood loss of 200 ml, requiring transfusion of 100 ml packed red blood cells. The patient was transferred to the Pediatric Intensive Care Unit, where she remained under observation for 48 hours without experiencing any seizures.
Discussion
The primary goal in the treatment of patients with epilepsy is to achieve adequate seizure control to improve their quality of life and prevent the deterioration associated with frequent and prolonged epileptic seizures over the years. Currently, drug-resistant seizures can be either cured or controlled through surgery.6
ECoG represents baseline cortical activity. It is similar to scalp EEG, but without the dispersion and attenuation of potentials caused by the scalp and skull. The ECoG waveform pattern varies depending on electrode placement, preexisting lesions, preoperative medications, and the presence of different anesthetic agents.7 Therefore, the choice of anesthetic drugs for maintenance must be based on their ability to alter cortical electrical activity, aid in the localization and delineation of the epileptogenic focus, and ultimately impact the patient’s prognosis.5 For anesthetic maintenance in the case presented, propofol and dexmedetomidine were selected for the aforementioned reasons.
Propofol is a gamma-aminobutyric acid agonist with a rapid onset and short half-life. It decreases cerebral oxygen consumption, intracranial pressure, and postoperative nausea and vomiting. Its effect on brain electrical activity is dual and dose-dependent, meaning that high doses can result in an undesirable flat EEG trace.8
Dexmedetomidine is an alpha-2 agonist with an average onset of 15 minutes and has hypnotic, anxiolytic, amnestic, and analgesic properties. It is considered the drug of choice because it does not cause respiratory depression, does not interfere with epileptogenic activity, and does not alter the EEG trace.9
Sufentanil was used as the opioid of choice, helping to attenuate the hemodynamic response to noxious stimuli in a manner similar to fentanyl. Although sufentanil induces respiratory depression by reducing the responsiveness of brainstem respiratory centers to increased CO₂ levels, it does not affect the electrocorticography trace when used at low doses or by infusion.5,10 Its elimination half-life is shorter in infants and children. The recommended plasma concentration ranges are: 0.8 ng/ml for intubation, 1–3 ng/ml for incision, 0.25–1 ng/ml for minor surgery, <0.2 ng/ml for spontaneous ventilation, and 0.2–0.4 ng/ml for postoperative analgesia.11
Conclusion
Epilepsy surgery is a complex procedure that requires a multidisciplinary team for proper management, including the participation of a neurologist, neurosurgeon, neurophysiologist, and anesthesiologist. Each team member must understand their specific objectives to achieve favorable outcomes.
The anesthesiologist plays a vital role, starting with a thorough preoperative evaluation, which serves as a guide for selecting the appropriate monitoring techniques. In addition, careful selection of anesthetic drugs is crucial to minimize interference with ECoG, as this is essential for accurate delineation of the epileptogenic focus and a higher success rate of the procedure.
TIVA remains the cornerstone of anesthesia in neurosurgical procedures. It allows for hemodynamic stability, a calm and rapid emergence for postoperative neurological assessment, and does not interfere with intraoperative electrophysiological monitoring, which is commonly used in these types of surgeries.12
In adults, the anesthetic technique known as asleep-awake-asleep is often used to delineate the seizure focus via ECoG and to identify eloquent brain areas through bipolar stimulation. The patient is then awakened to assess the function of the stimulated area.5 This technique can also be used in older, cooperative pediatric patients; however, due to the patient’s age and severe neurodevelopmental delay, she was not a candidate for this approach. Upon reviewing the literature, we found very few reported cases with the characteristics previously described in our patient, so we consider this publication useful.
Acknowledgements
None.
Conflict of interest
The authors declare no conflict of interest.
References
- Petre C, Bartuluchi M, Vázquez C, et al. Outcomes of epilepsy surgery in tuberous sclerosis. Rev Argent Neuroc. 2008;22(3):134–135.
- Docampo J. Tuberous sclerosis: Evaluation of intracranial lesions. Revista Argentina de Radiología. 2013;77(4):275–283.
- Marchant JK, Nardiello MM, Henríquez AA. Halogenated anesthesia or total intravenous anesthesia in neurosurgery? Revista Chilena de Anestesia. 2021;50(4):576–581.
- Smeyers Durá P, De Santos MT. Tuberous sclerosis complex. Protoc Diagn Ter Pediatr. 2022;1:353–359.
- Rojas-Ávila IA, Cárdenas-España M, Cruz-Cruz EF, et al. The role of the anesthesiologist in epilepsy surgery. Anales Médicos (México). 2020;65(3):214–223.
- Functional Surgery Group of the Spanish Society of Neurosurgery (SENEC). Guidelines for the surgical treatment of the movement disorders and epilepsy. Neurocirugía. 2009;20(4):329–334.
- Chui J, Manninen P, Valiante TA, et al. Anesthetic considerations of intraoperative electrocorticography during epilepsy surgery. Anesth Analg. 2013;117(2):479–486.
- San-Juan D, Chiappa KH, Cole AJ. Propofol and the electroencephalogram. Clin Neurophysiol. 2010;121(7):998–1006.
- Chaitanya G, Arivazhagan A, Sinha S, et al. Dexmedetomidine anesthesia enhances spike generation during intraoperative electrocorticography: A promising adjunct for epilepsy surgery. Epilepsy Research. 2015;109(1):65–71.
- Bustinza AC, Torrent A. TCI of sufentanil vs. TCI of remifentanil: Any advantages? AnestesiaR. 2017;9(6):3.
- Ortiz JR, Lora-Tamayo JI. Total Intravenous Anesthesia: Basic Principles. 2nd ed. Chapter 5; 2009. 123–145 pp.
- Llorente-Mariñez GM. Why TIVA in neurosurgery: Is it a good option? Revista Mexicana de Anestesiología. 2014;37(Suppl 1):369–373.

